If the average person was asked to do a mass balance on a system, they might not quite understand what is required. However, for chemical engineers and industrial process chemists this is something learned quite early in their curriculum. While it is a tool often used by chemical engineers, there is no reason why it should not be part of any processor’s toolbox.
For processes involving chemical reactions, the mass balance is written out as:
INPUT + GENERATION = OUTPUT + ACCUMULATION + CONSUMPTION
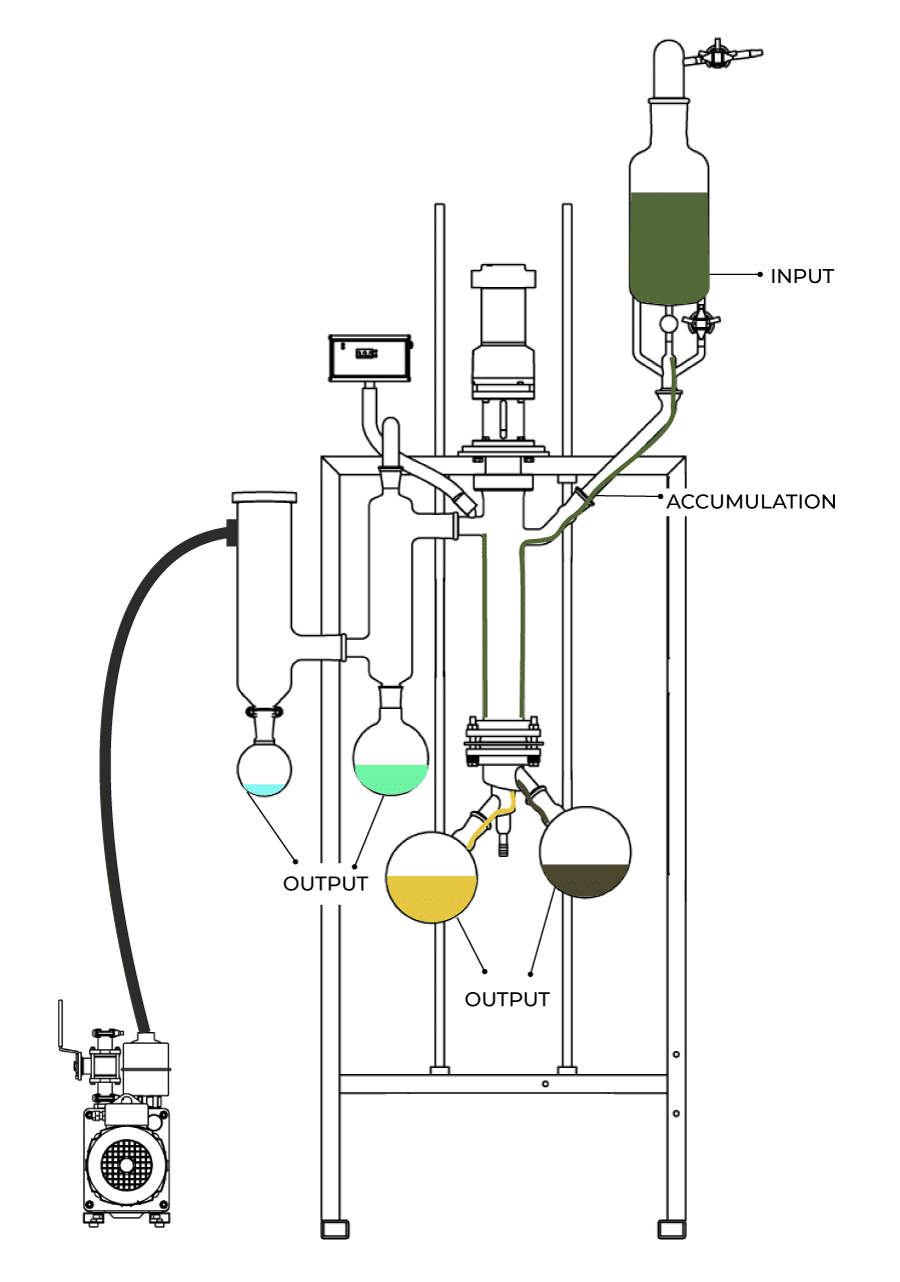
However, when you are just using separation equipment (such as a wiped-film molecular still for distillation), the equation gets simplified to:
INPUT = OUTPUT + ACCUMULATION
Then, by defining the boundaries of a system, it becomes possible to check where your material is going which can allow you to make better decisions and optimize your process.
Let’s assume you are running a wiped-film molecular still to create CBD distillate. The INPUT to the still will be the feed material (the crude). The OUTPUT of this separation will be terpenes, residue, and CBD distillate. The ACCUMULATION would be any residual material left in the still. There are other outputs; the ethanol collected in the cold trap and any material that is lost through the vacuum pump, however, to simplify this exercise we will lump these into the accumulation and simply define them all as material loss.
While losses to accumulation are important, wiped film stills do not have miles of process piping, so overall this should not be a major concern on the equipment. However, by adding some analytical data to the mass balance much can be learned about your operation.

Image source: Vext Science www.vextscience.com
Example:
Prior to distillation, you have your crude CBD extract analyzed and the lab results come back indicating the material is 65% CBD by mass. You load the feed flask on your system with 2,000 grams of the material. You perform your first distillation and remove 160 grams of terpenes. On your second pass you get 1,155 grams of distillate and 635 grams of residue. When the lab results on your distillate come back, they show 90% CBD by mass. This seems good, but a mass balance will allow you to best review this.
For a Very Simple Mass Balance, You Have:
mcrude = mterpenes + mdistillate + mresidue + mloss (1)
Where:
mcrude = mass of crude
mterpenes = mass of terpenes collected
mdistillate = mass of distillate collected
mresidue = mass of residue collected
mloss = material lost (to accumulate, transfer, etc.)
This yields 50 grams of material that is considered lost. Which could either be escaped ethanol or material that sticks as residual in the system (a common occurrence with high viscosity fluids).
Since there is not a large amount of material that was lost, it is now time to look at the amount of CBD you have recovered, also known as your yield. First, you must determine how much CBD is present to start, using equation (2):
mCBD-C = mcrude * CCBD-C (2)
Where:
mCBD-C = mass of CBD in crude
mcrude = mass of crude
CCBD-C = CBD Concentration in crude (mass %)
This equation shows that 1,300 grams of CBD are present in your crude. A similar equation can be used to determine the amount of CBD in your distillate:
mCBD-D = mdistillate * CCBD-D (3)
Where:
mCBD-D = mass of CBD in distillate
CCBD-D = CBD Concentration in distillate (mass %)
Leading to a total of 1,039.5 grams of CBD in your distillate. To calculate the yield of your process you then use Equation 4:
% Yield = mCBD-D ÷ mCBD-C (4)
The percent yield from this distillation is approximately 80%, which would be considered quite sufficient. By further calculation, you can also estimate the amount of CBD that remains in your residue. We will assume that the 50 grams of lost material were at 65% CBD content, like the crude, meaning that 32.5 g of CBD where “lost”. By Equations 5 and 6, you can assume your remaining residue contains 228 grams of CBD or is 36% CBD (mass %):
mCBD-R = mCBD-C – mCBD-D – mCBD-L (5)
Where:
mCBD-R = mass of CBD in residue
mCBD-L = mass of CBD lost
CCBD–R = mCBD-R ÷ mresidue (6)
Where:
CCBD–R = CBD concentration in residue (mass %)
A great amount of processing in the cannabis industry is performed without much attention to mass balancing and utilizing before/after analyses between process steps. In many of these cases, the company will get by, obtaining adequate purities and (often unknown) yields. A somewhat careful operator will often be able to “get the job done”. However, consistent utilization of the principles of mass balancing, together with obtaining and studying analyses of every batch and run will allow an OK operator to become an excellent operator. This person will be able to plan parameters as well as strategies for each run, resulting in optimal purity and yield, leading to lower costs, better product quality, at better pricing, all eventually leading to greater profit for their company than their competitors.

